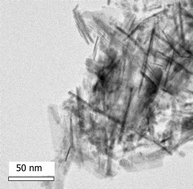
Transition metal chlorides are reacted with lithium amide or ammonia under solvothermal conditions in benzene at temperatures up to 550 °C. The products are metal nitrides with particle sizes of a few nm. VN, NbN, CrN, MoN and WN form with a cubic rocksalt-type structure, whilst Ta3N5 adopts the known orthorhombic structure. Products often contain carbon due to solvent decomposition, the carbon content is higher when ammonia is the nitrogen source, and varied to some extent from metal to metal. Analytical data shows nitrogen-deficient carbonitride compositions. Most samples crystallise with partially aggregated, regular crystallites. Some crystallise with a nanorod morphology and this was most pronounced in Ta3N5, which forms high aspect ratio, single crystal nanorods when synthesised with ammonia.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...