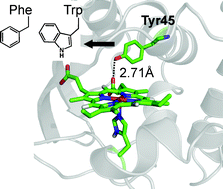
In a previous study, we constructed a prototype thermally tolerant artificial peroxidase from an electron transfer protein, cytochrome c552 from Thermus thermophilus, and demonstrated that engineering of proteins from thermophiles could be a promising methodology to produce artificial enzymes for practical use. In the present study, further improvement of the prototype (the V49D/M69A mutant) in enzymatic activity and thermal tolerance has been achieved by successive modifications based on detailed analyses of an active intermediate formed in the peroxidase reaction. Spectroscopic studies revealed that the major active intermediate of V49D/M69A was an oxo-ferryl heme with a protein radical predominantly localized on Tyr45. The magnetic power saturation measurement in EPR studies showed little magnetic coupling between the oxo-ferryl heme and the tyrosyl radical. This result indicated that the oxo-ferryl heme and the tyrosyl radical served as isolated oxidants. Kinetics studies indicated that the isolated oxo-ferryl heme component in the active intermediate could be a precursor to heme degradation by the reaction with H2O2. Replacement of Tyr45 with phenylalanine on V49D/M69A resulted in delocalization of the radical over the protein and increased magnetic coupling between the oxo-ferryl heme and the protein radical in the intermediate. The stronger magnetic coupling between the oxo-ferryl heme and the radical was achieved by replacement of Tyr45 with tryptophan, which was similar to a tryptophanyl radical found in active intermediates of some catalase-peroxidases. The protein radical intermediates of the Tyr45 mutants exhibited relatively higher reactivity to an organic substrate than H2O2 in comparison to the basal mutant, V49D/M69A, which was preferable to suppress the heme degradation process. This was reflected in the improved enzymatic activity and thermal tolerance observed for the Tyr45 mutants in the peroxidase reaction.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...