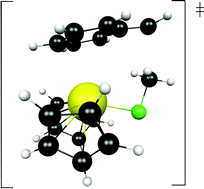
The experimental reaction between [1,2,4-(Me3C)3C5H2]2CeCH2Ph and CH3X, X = F, Cl, Br, and I, yields the metathetical exchange products, [1,2,4-(Me3C)3C5H2]2CeX and CH3CH2Ph. The reaction is complicated by the equilibrium between the benzyl derivative and the metallacycle [1,2,4-(Me3C)3C5H2][(Me3C)2C5H2C(CH3)2CH2]Ce, plus toluene since the metallacycle reacts with CH3X. Labelling studies show that the methyl group of the methylhalide is transferred intact to the benzyl group. The mechanism, as revealed by DFT calculations on (C5H5)2CeCH2Ph and CH3F, does not proceed by way of a four-center mechanism, a σ-bond metathesis, but by a lower barrier process involving a haptotropic shift of the Cp2Ce fragment so that at the transition state the para-carbon of the benzene ring is attached to the Cp2Ce fragment while the CH2 fragment of the benzyl group attacks CH3F that is activated by coordination to the metal ion. As a result the mechanism is classified as an associative interchange process.

 Please wait while we load your content...
Please wait while we load your content...