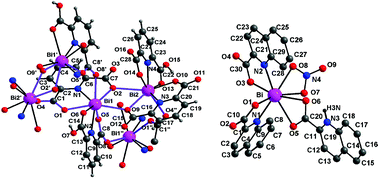
Three crystalline compounds, [Bi(2-O2C–C5H4N)3]n (1), {Bi[(2,6-O2C)2C5H3N)][(2-HO2C-6-O2C)C5H3N]·H2O}n (2) and Bi(O2CC9H6N)2(O3N)(O2CC9H6NH)·2H2O (3) have been prepared by simple reactions in aqueous medium using the readily available bismuth nitrate and the corresponding acids, picolinic acid, dipicolinic acid and quinaldic acid. While 1 and 2 are coordination polymers with bismuth in tricapped trigonal prismatic and dodecahedral environments, compound 3 is a monomeric species with dodecahedral geometry at bismuth. Compound 1 represents a second crystalline form of a recently reported structure with subtle differences in bond parameters, and highlights the flexibility in structural motifs during crystallization. Compound 2 involves skeletons with dimeric [Bi2O2] and trimeric [BiOCOBiOBiOCO] moieties. In 3, while the N-protonated carboxylate forms a four-membered chelate ring with bismuth, the other two carboxylates form five-membered ring chelates with the nitrate accounting for the remaining two sites again as a chelate. TGA studies are consistent with the presence of non-coordinated water in 2 and 3. Compounds 1 and 2, although insoluble in most of the organic solvents and water, are readily soluble in dilute hydrochloric acid.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...