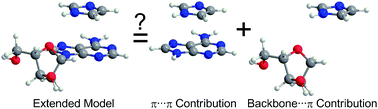
The (gas-phase) MP2/6-31G*(0.25) π⋯π stacking interactions between the five natural bases and the aromatic amino acids calculated using (truncated) monomers composed of conjugated rings and/or (extended) monomers containing the biological backbone (either the protein backbone or deoxyribose sugar) were previously compared. Although preliminary energetic results indicated that the protein backbone strengthens, while the deoxyribose sugar either strengthens or weakens, the interaction calculated using truncated models, the reasons for these effects were unknown. The present work explains these observations by dissecting the interaction energy of the extended complexes into individual backbone⋯π and π⋯π components. Our calculations reveal that the total interaction energy of the extended complex can be predicted as a sum of the backbone⋯π and π⋯π components, which indicates that the biological backbone does not significantly affect the ring system through π-polarization. Instead, we find that the backbone can indirectly affect the magnitude of the π⋯π contribution by changing the relative ring orientations in extended dimers compared with truncated dimers. Furthermore, the strengths of the individual backbone⋯π contributions are determined to be significant (up to 18 kJ mol−1). Therefore, the origin of the energetic change upon model extension is found to result from a balance between an additional (attractive) backbone⋯π component and differences in the strength of the π⋯π interaction. In addition, to understand the effects of the biological backbone on the stacking interactions at DNA–protein interfaces in nature, we analyzed the stacking interactions found in select DNA–protein crystal structures, and verified that an additive approach can be used to examine the strength of these interactions in biological complexes. Interestingly, although the presence of attractive backbone⋯π contacts is qualitatively confirmed using the quantum theory of atoms in molecules (QTAIM), QTAIM electron density analysis is unable to quantitatively predict the additive relationship of these interactions. Most importantly, this work reveals that both the backbone⋯π and π⋯π components must be carefully considered to accurately determine the overall stability of DNA–protein assemblies.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...