An improved method to measure the rate of vaporisation and thermal decomposition of high boiling organic and ionic liquids by thermogravimetrical analysis†
Abstract
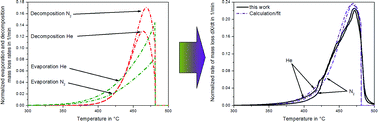
The vapour pressure and the thermal stability of liquids are important material properties. For high boiling organic and ionic liquids (ILs), the determination of these properties is laborious and it is not easy to discriminate between

 Please wait while we load your content...
Please wait while we load your content...