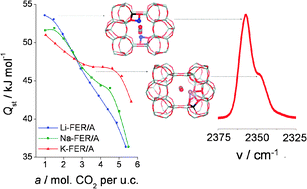
The adsorption of CO2 in Li-, Na-, and K-FER was investigated by a combination of volumetric adsorption experiments, FTIR spectroscopy, and density functional theory. Experimental isosteric heats of CO2, Qst, depend significantly on the cation size, cation concentration, and on the amount of adsorbed CO2. The differences observed in experimentally determined isosteric heats were interpreted at the molecular level based on good agreement between experimental and calculated characteristics. The highest interaction energies were found for CO2 adsorbed on so-called “dual cation sites” in which CO2 is bridged between two alkali metal cations. The formation of CO2 adsorption complexes on dual cation sites is particularly important on Na-FER and K-FER samples with higher cation concentration. On the contrary, the differences in Qst observed for Li-FER samples are due to the changes in the Li+ coordination with the framework. The DFT/CC calculations show that the dispersion interactions between CO2 and the zeolites framework are rather large (about −20 kJ mol−1).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...