A four-dimensional compound-model morphed potential for the OC:HBr complex
Abstract
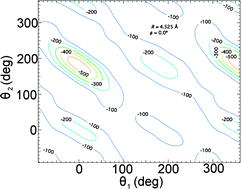
A parameterized compound-model morphed intermolecular potential energy surface has been generated for the dimer OC:HBr. This morphed potential is determined by fitting experimentally available gas phase spectroscopic data and found to have a global minimum with a well depth of 564(5) cm−1 and linear 16O12C–H79Br geometry having center of mass to center of mass distance R = 4.525(7) Å. The linear isomers 12C16O–H79Br and 16O12C–79BrH are determined with a corresponding well depth of 273(7) and 269(2) cm−1 having R = 4.35(4) and 4.24(3) Å, respectively. This results in a ΔE of 293(9) cm−1 between the global potential energy minimum and the minima in the two higher energy isomers. The generated potential is compared with the corresponding OC:HCl morphed potential. Differences in the morphing parameters are attributed to different contributions to the interaction energy. It is found that the counterpoise method successfully corrected the basis set superposition error in OC:HCl, but was under corrected by 16(7)% in OC:HBr.

 Please wait while we load your content...
Please wait while we load your content...