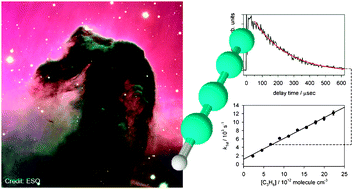
The kinetics of the reactions of the linear butadiynyl radical, C4H (CCCCH), with a variety of unsaturated hydrocarbons have been studied over the temperature range of 39–300 K using a CRESU (Cinétique de Réaction en Ecoulement Supersonique Uniforme, or reaction kinetics in uniform supersonic flow) apparatus combined with the pulsed laser photolysis–laser induced fluorescence technique. The rate coefficients for all the reactions studied are found to all be in excess of 10−10 cm3 molecule−1 s−1 over the entire temperature range. They can be fitted with the following expressions (valid from 39 K to 300 K, with RMS deviations of the experimental points from the predicted values shown, to which should be added 10% possible systematic error) for reaction of C4H with alkenes: kC2H4 = (1.95 ± 0.17) × 10−10 (T/298 K)−0.40 exp(9.4 K/T) cm3 molecule−1 s−1;kC3H6 = (3.25 ± 0.12) × 10−10 (T/298 K)−0.84 exp(−48.9 K/T) cm3 molecule−1 s−1;k1-C4H8 = (6.30 ± 0.35) × 10−10 (T/298 K)−0.61 exp(−65.0 K/T) cm3 molecule−1 s−1,for reaction of C4H with dienes:kC3H4 = (3.70 ± 0.34) × 10−10 (T/298 K)−1.18 exp(−91.1 K/T) cm3 molecule−1 s−1;k1,3-C4H6 = (5.37 ± 0.30) × 10−10 (T/298 K)−1.25 exp(−116.8 K/T) cm3 molecule−1 s−1,and for reaction of C4H with alkynes: kC2H2 = (1.82 ± 0.19) × 10−10 (T/298 K)−1.06 exp(−65.9 K/T) cm3 molecule−1 s−1;kC3H4 = (3.20 ± 0.08) × 10−10 (T/298 K)−0.82 exp(−47.5 K/T) cm3 molecule−1 s−1;k1-C4H6 = (3.48 ± 0.14) × 10−10 (T/298 K)−0.65 exp(−58.4 K/T) cm3 molecule−1 s−1.Possible reaction mechanisms and product channels are discussed in detail for each of these reactions. Potential implications of these results for models of low temperature chemical environments, in particular cold interstellar clouds and star-forming regions, are considered.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...