A numerical investigation into possible mechanisms by that the A629P mutant of ATP7A causes Menkes Disease†
Abstract
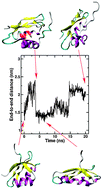
We study in silico possible mechanisms by that the A629P mutant of ATP7A causes Menkes Disease. Our results indicate that the mutation does not have appreciable affects on the stability of

 Please wait while we load your content...
Please wait while we load your content...