Synthesis, structural and conformational analysis of a 3 × 3 isomer grid based on nine methyl-N-(pyridyl)benzamides†
Abstract
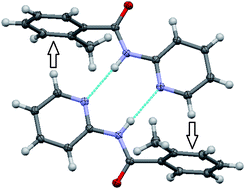
A 3 × 3 isomer grid of nine methyl-N-(pyridyl)benzamides (C13H12N2O) as Mxx (x = para-/meta-/ortho-) has been synthesized and characterised to evaluate and correlate structural relationships from both ab initio calculations (gas-phase, PCM-SMD model solvated forms) and the solid-state. The effect of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Camide in Mpm. The N–H⋯N interactions form
Camide in Mpm. The N–H⋯N interactions form

 Please wait while we load your content...
Please wait while we load your content...