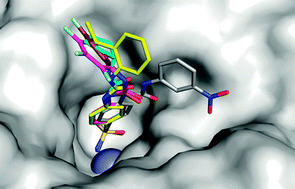
Selective hydrophobic pocket binding observed within the carbonic anhydrase II active site accommodate different 4-substituted-ureido-benzenesulfonamides and correlate to inhibitor potency†
Abstract
4-Substituted-ureido benzenesulfonamides showing inhibitory activity against carbonic anhydrase (CA, EC 4.2.1.1) II between 3.3–226 nM were crystallized in complex with the

 Please wait while we load your content...
Please wait while we load your content...