Kinetic resolution of trans-cycloalkane-1,2-diols via Steglich esterification†
Abstract
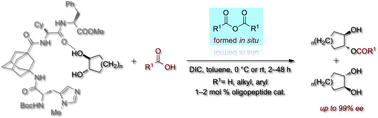
We describe the efficient and highly enantioselective kinetic resolution of trans-cycloalkane-1,2-diols utilizing an enantioselective Steglich reaction with a variety of carboxylic acids that form the corresponding anhydrides in situ.

 Please wait while we load your content...
Please wait while we load your content...