Extending the reliability and applicability of B3LYP
Abstract
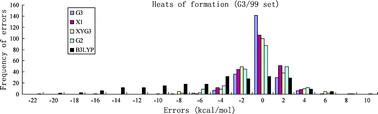
B3LYP is by far the most popular density functional in chemistry. Nevertheless, there is growing evidence, showing that B3LYP (1) degrades as the system becomes larger, (2) underestimates reaction barrier heights, (3) yields too low bond dissociation enthalpies, (4) gives improper isomer energy differences, and (5) fails to bind van der Waals systems, etc.

 Please wait while we load your content...
Please wait while we load your content...