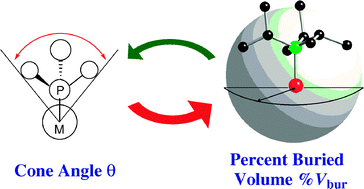
Percent buried volume for phosphine and N-heterocyclic carbeneligands: steric properties in organometallic chemistry
Abstract
Electronic and steric
- This article is part of the themed collection: ChemComm 60th Anniversary Historic Papers from the United Kingdom

 Please wait while we load your content...
Please wait while we load your content...