Development and validation of an LC-MS/MS method for determination of phencyclidine in human serum and its application to human drug abuse cases
Abstract
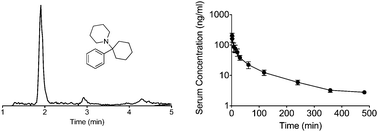
A new analytical method was developed and validated for the rapid determination of phencyclidine (PCP) in human blood and serum. Rapid chromatographic separation decreased the analysis time relative to standard

 Please wait while we load your content...
Please wait while we load your content...