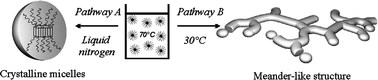
We studied the morphology of micelles formed by a well-defined poly(1,2-butadiene)-block-poly(ethylene oxide) diblock copolymer (PB-b-PEO). Dissolved in n-heptane at 70 °C, that is, above the melting point of PEO, spherical micelles are formed due to the selectivity of the solvent for the PB-block. If the solutions are cooled down to low temperatures, the liquid PEO-block crystallizes within the cores of the spherical micelles that remain stable. If, however, the solutions are quenched to 30 °C, the spherical micelles aggregate to a novel meander-like structure within several minutes. In its final state, the meander-like super-structure is crystalline, as revealed by time-resolved wide-angle X-ray scattering. The super-structure is shown to result from crystallization-induced aggregation of spherical micelles. Moreover, crystallization leads to well-defined angles between subsequent aggregating units. A quantitative Avrami-type analysis of the crystallization kinetics demonstrates that the formation of the meander-type structure resembles a 2D growth process combined with a breakout crystallization, showing an Avrami-exponent of 2.5. In contrast to this, crystallization at low temperatures resembles a confined crystallization with a low Avrami-exponent of 0.7. All data demonstrate that the morphology of block copolymers having a crystallizable block can be switched by the competition of aggregation and crystallization.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...