The effective role of positive charge saturation in bioluminescence color and thermostability of firefly luciferase
Abstract
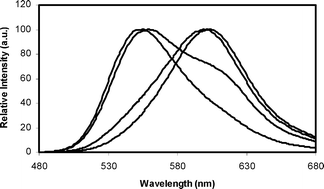
Luciferases are the enzymes that catalyze the reactions that produce light in bioluminescence. The bioluminescence color of firefly luciferases is determined by the luciferase structure and assay conditions. Amongst different beetle luciferases, those from phrixothrix rail-road worm with a unique additional residue (Arg353) emit red bioluminescence color naturally. Insertion of Arg356 in Lampyris turkestanicus luciferase changed the emitted light to red with a bimodal bioluminescence spectrum. By insertion and substitution of positively-charged residues, different specific mutation (E354R/Arg356, E354K/Arg356, E354R, E354K) lead to changes of the bioluminescence color. Bioluminescence emission spectra indicate that substitution of E354 by R along with insertion of Arg356 produces a luciferase that emits red light with a single peak bioluminescence spectrum. The comparison of mutants with native luciferase shows that mutations of firefly luciferase resulted in structural and functional thermostability. Comparative study of native and mutant luciferase (E354R/Arg356) by intrinsic and extrinsic fluorescence, CD spectropolarimetry, and homology modeling revealed mutation brought about an increase in content of secondary structure and globular compactness of L. turkestanicus luciferase. On the other hand, pKa of amino acids in the flexible loop decreased upon introducing of positive charges.

 Please wait while we load your content...
Please wait while we load your content...