Neighbouring group participation vs. addition to oxacarbenium ions: studies on the synthesis of mycobacterial oligosaccharides†
Abstract
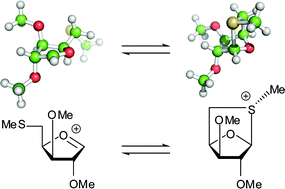
Neighbouring group participation is frequently used to control the stereoselectivity of chemical reactions. Herein, we investigate the use of neighbouring group participation for the synthesis of disaccharides incorporating the mycobacterial sugar methylthioxylose. A bicyclic thioglycoside was activated by methylation to generate a methylsulfonium group that would act both as the anomeric leaving group, and also provide the methylsulfide group in the product. Model reactions indicated that the bicyclic intermediate would also act as a participating group to direct the acceptor alcohol to the lower α-face of the sugar. While the key sulfonium intermediate could be detected in the reaction mixture, the glycosylation reaction proceeded with moderate stereoselectivity, apparently via an SN1-type mechanism. Density functional theory calculations were used to compare our methylthioxylose sulfonium ion with a trans-decalin-like sulfonium ion described by Boons and co-workers to be an α-directing participating group (J. Am. Chem. Soc. 2005, 127, 12090). Our studies show that even where a bicyclic sulfonium ion can be detected in the reaction mixture, caution should be applied before invoking it as an intermediate on the reaction pathway.

 Please wait while we load your content...
Please wait while we load your content...