The aminocoumarins: biosynthesis and biology
Abstract
Covering: up to 2009
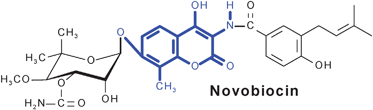
The aminocoumarin antibiotics are characterized by their 3-amino-4,7-dihydroxycoumarin moiety. This family of antibiotics comprises highly potent gyrase inhibitors, including novobiocin and the structurally related compounds clorobiocin and coumermycin A1. These compounds interact with the B subunit of bacterial gyrase and inhibit ATP-dependent supercoiling of DNA. The structurally more complex simocyclinone D8, which contains two polyketide moieties, inhibits gyrase by a completely different mechanism, i.e.via interaction with the A subunit. Rubradirin and its aglycone, which contain an ansamacrolide moiety, interfere with protein or RNA synthesis, respectively. The biosynthetic gene clusters of all five aminocoumarin antibiotics have been identified, and the gene functions have been studied by genetic and biochemical methods. The biosynthesis of novobiocin and clorobiocin is now one of the best-understood pathways of secondary metabolism in streptomycetes.

 Please wait while we load your content...
Please wait while we load your content...