Photoinduced electron transfer in a fullerene–oligophenylenevinylene dyad
Abstract
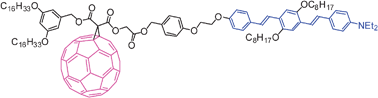
A dialkylamino-subtituted oligophenylenevinylene (OPV) derivative bearing a
* Corresponding authors
a
Laboratoire de Chimie des Matériaux Moléculaires, Ecole Européenne de Chimie, Polymères et Matériaux, Université de Strasbourg et CNRS (UMR 7509), 25 rue Becquerel, 67087 Strasbourg Cedex 2, France
E-mail:
nierengarten@chimie.u-strasbg.fr
b
Laboratoire de Chimie de Coordination du CNRS, 205 route de Narbonne, 31077 Toulouse Cedex 4, France
E-mail:
Beatrice.Delavaux-Nicot@lcc-toulouse.fr
c
Istituto per la Sintesi Organica e la Fotoreattività, Consiglio Nazionale delle Ricerche, via Gobetti 101, 40129 Bologna and Via Borsari 46 44100 Ferrara, Italy
E-mail:
armaroli@isof.cnr.it
A dialkylamino-subtituted oligophenylenevinylene (OPV) derivative bearing a

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
A. Gégout, J. L. Delgado, J. Nierengarten, B. Delavaux-Nicot, A. Listorti, C. Chiorboli, A. Belbakra and N. Armaroli, New J. Chem., 2009, 33, 2174 DOI: 10.1039/B9NJ00259F
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
