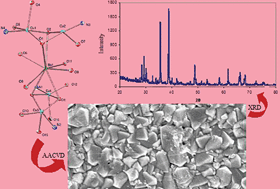
Heterobimetallic molecular precursors [Ba(dmap)4Cu4(OAc)6·THF] (1) and [Ba(dmap)4Cu4(TFA)6·THF] (2) [dmap = N,N-dimethylaminopropanolate, OAc = acetate and TFA = trifluoroacetate] for the deposition of barium–copper composite oxide thin films, were prepared by the interaction of Ba(dmap)2 with Cu(OAc)2 for 1 and Cu(TFA)2 for 2, in THF. Both heterobimetallic complexes were characterized by melting point, elemental analysis, FT-IR spectroscopy, mass spectrometry and single crystal X-ray diffraction. X-Ray crystallography shows that complex 1 crystallizes in the orthorhombic space groupP212121 with the cell dimensions a = 11.2621(11) Å, b = 18.2768(17) Å and c = 24.541(2) Å, while complex 2 crystallizes in the monoclinic space groupC2/c with a = 23.9288(14) Å, b = 19.8564(12) Å, c = 25.5925(15) Å and β = 112.4390(10)°. Thermal gravimetric analysis shows that both complexes 1 and 2 undergo controlled thermal decomposition at 450 °C and 400 °C, respectively, to give mixed metal oxide composite thin films. Scanning electron microscopy (SEM), energy dispersive X-ray (EDX) and X-ray powder diffraction (XRD) analyses of the thin films suggest the formation of good quality crystalline thin films of BaCuO2–CuO composites from both 1 and 2, with average grain sizes of 105 to 175 nm and 110 to 205 nm, respectively.

 Please wait while we load your content...
Please wait while we load your content...