Interaction of thimerosal with proteins—ethylmercuryadduct formation of human serum albumin and β-lactoglobulin A
Abstract
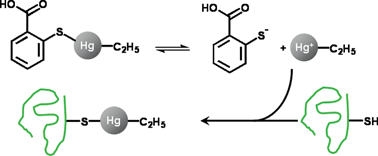
The interaction of
* Corresponding authors
a
University of Münster, Institute of Inorganic and Analytical Chemistry, Corrensstr. 30, Münster, Germany
E-mail:
uk@uni-muenster.de
Fax: +49 251 83 36013
Tel: +49 251 83 33141
b European Virtual Institute for Speciation Analysis, Mendelstr. 11, Münster, Germany
The interaction of

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
