The serotonergic system has been implicated in emotional and cognitive function. In particular, 5-HT2A (5-hydroxytrytamine receptor 2A) is attributed to a number of disorders like schizophrenia, depression, eating disorders and anxiety. 5-HT2A, being a GPCR (G-protein coupled receptor), is important in the pharmaceutical industry as a proven target for these disorders. Despite their extensive clinical importance, the structural studies of this protein is lacking due to difficulties in determining its crystal structure.
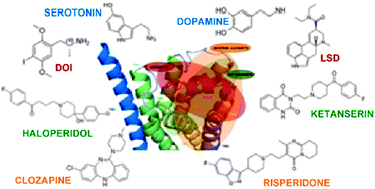
We have performed sequence analysis and molecular modeling of 5-HT2A that has revealed a set of conserved residues and motifs considered to play an important role in maintaining structural integrity and function of the receptor. The analysis also revealed a set of residues specific to the receptor which distinguishes them from other members of the subclass and their orthologs. Further, starting from the model structure of human 5-HT2A receptor, docking studies were attempted to envisage how it might interact with eight of its ligands (such as serotonin, dopamine, DOI, LSD, haloperidol, ketanserin, risperidone and clozapine). The binding studies of dopamine to 5-HT2A receptor can bring up better understanding in the etiology of a number of neurological disorders involving both these two receptors. Our sequence analysis and study of interactions of this receptor with other ligands reveal additional residue hotspots such as Asn 363 and Tyr 370. The function of these residues can be further analyzed by rational design of site-directed mutagenesis. Two distinct binding sites are identified which could play important roles in ligand binding and signaling.

 Please wait while we load your content...
Please wait while we load your content...