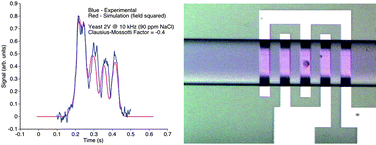
In biomedical applications ranging from the study of pathogen invasion to drug efficacy assays, there is a growing need to develop minimally invasive techniques for single-cell analysis. This has inspired researchers to develop optical, electrical, microelectromechanical and microfluidic devices for exploring phenomena at the single-cell level. In this work, we demonstrate an electrical approach for single-cell analysis wherein a 1.6 GHz microwave interferometer detects the capacitance changes (ΔC) produced by single cells flowing past a coplanar interdigitated electrode pair. The experimental and simulated capacitance changes generated by yeast cells are in close agreement. By using the capacitance changes of uniform polystyrene spheres (diameter = 5.7 µm) for calibration purposes, we demonstrate a 0.65 aF sensitivity in a 10 ms response time.
Using an RC circuit, a low frequency sinusoidal potential is simultaneously superimposed on the electrode pair to generate a dielectrophoretic force that translates cells. Specifically, when yeast cells suspended in a solution of 90 ppm NaCl in deionized water are exposed to 10 kHz and 3 MHz potentials (ranging from 1–3 Vpp), they experience negative and positive dielectrophoresis, respectively. The corresponding changes in cell elevation above the interdigitated electrodes are detected using the asymmetry of the capacitance signature produced by the cell. Cell elevation changes can be detected in less than 80 ms. The minimum detectable change in elevation is estimated to be 0.22 µm. This approach will have applications in rapid single-cell dielectrophoretic analysis, and may also prove useful in conjunction with impedance spectroscopy.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...