Basic principles of electrolyte chemistry for microfluidic electrokinetics. Part II:† Coupling between ion mobility, electrolysis, and acid–base equilibria‡
Abstract
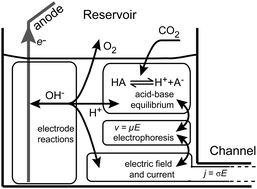
We present elements of electrolyte dynamics and electrochemistry relevant to microfluidic electrokinetics experiments. In Part I of this two-paper series, we presented a review and introduction to the fundamentals of acid–base chemistry. Here, we first summarize the coupling between acid–base equilibrium chemistry and electrophoretic mobilities of electrolytes, at both infinite and finite dilution. We then discuss the effects of electrode reactions on microfluidic electrokinetic experiments and derive a model for pH changes in microchip reservoirs during typical direct-current electrokinetic experiments. We present a model for the potential drop in typical microchip
- This article is part of the themed collection: Fundamental Principles and Techniques in Microfluidics

 Please wait while we load your content...
Please wait while we load your content...