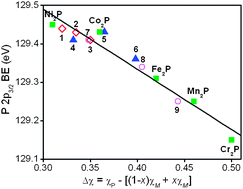
Mixed-metal variants (Ni1−xM′x)2P (M′ = Cr, Fe, Co; 0 ≤ x ≤ 1) of the binary phosphide Ni2P, a well-known hydrodesulfurization and hydrodenitrogenation catalyst, have been examined by X-ray photoelectron (XPS) and absorption (XANES) spectroscopy. The P 2p3/2 binding energies and K-edge absorption energies in (Ni1−xCrx)2P, (Ni1−xFex)2P, and (Ni1−xCox)2P are lower than in Ni2P, with the shifts becoming more pronounced with greater difference in electronegativity between P and Ni/M′. These shifts are influenced primarily by intraatomic effects. However, secondary interatomic effects are also implicated in the Ni 2p3/2XPS spectra, which show enhanced satellite intensities, and in the Ni K- and L-edge absorption spectra, indicating a charge transfer from M′ to the more electronegative Ni atoms. This phenomenon was confirmed in the metal L-edge XANES spectra, which revealed lower Ni and higher M′ L-edge absorption energy as the degree of charge transfer increases. The Ni K-edge XANES spectra also show a pre-edge feature whose intensity decreases from Ni2P to (Ni0.8Cr0.2)2P, supporting the occurrence of charge transfer from Cr to Ni.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...