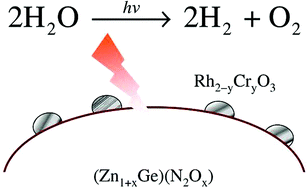
Enhancement of photocatalytic activity of zinc-germanium oxynitride solid solution for overall water splitting under visible irradiation
Abstract
Overall
- This article is part of the themed collections: Solar Energy Conversion and Solar energy conversion

 Please wait while we load your content...
Please wait while we load your content...