Halide binding in laterally non-symmetric aza-oxa cryptands through N/O/C–H⋯halide interactions with characterization of small water clusters†
Abstract
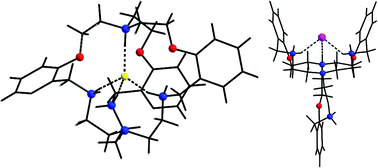
The structural study of halide complexes has been performed on two laterally non-symmetric aza-oxa cryptands Loo and Lmm having different cavity dimensions. For the fluoride complex of Loo, the ‘oxa’ end bridgehead N atom gets protonated to pull the internal fluoride towards the ‘oxa’ end. While bromide remains outside the cavity either by being sandwiched between the opposite-side directed arms of the two different moieties or by being incorporated into the space provided by the two same-side directed arms of a single Loo. In comparison, Lmm having a higher cavity dimension can accommodate chloride and bromide in addition to fluoride in its cavity. While Loo changes its conformation for its bromide complex attaining a ‘Y’-shaped architecture, cryptand Lmm retains its symmetrical pinwheel shape viewed down the C3 axis joining the two bridgehead

 Please wait while we load your content...
Please wait while we load your content...