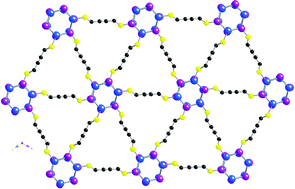
Treatment of CuI with the flexible PhS(CH2)4SPh dithioether ligand in MeCN solution affords the strongly luminescent metal–organic 2D coordination polymer [Cu4I4{μ-PhS(CH2)4SPh}2]n (1). The interpenetrated 2D network of 1 is built upon by Cu4(μ3-I)4 cubane-like clusters as secondary building units (SBUs), which are interconnected via bridging 1,4-bis(phenylthio)butane ligands. In contrast, the auto-assembly reaction of the unsaturated semi-flexible PhSCH2C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH2SPh ligand with CuI results in formation of the 3D metallopolymer [(Cu6I6){μ-PhSCH2C
CCH2SPh ligand with CuI results in formation of the 3D metallopolymer [(Cu6I6){μ-PhSCH2C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH2SPh}3]n (2). The SBUs of luminescent 2 consist of discrete Cu6(μ3-I)6 hexagon prisms, which are coordinated with bridging 1,4-bis(phenythio)butyne ligands via Cu–S bonds. Contrary to the other rare literature-known examples of metallopolymers incorporating Cu6X6 SBUs as connecting nodes, the Cu⋯Cu interactions of 2 [2.8484(6) Å] are markedly shorter, being close to the sum of the Van der Waals radii of two Cu atoms (∼2.8 Å). The photophysics of these compounds, which exhibit reversible luminescence thermochromism, has been investigated in detail. The solid-state luminescence spectra of 1 and 2 feature at room temperature intense emissions around 560 and 555 nm, respectively. The luminescence properties of the unusual Cu6(μ3-I)6 hexagon prism motif are rationalized by means of DFT and TDDFT computations.
CCH2SPh}3]n (2). The SBUs of luminescent 2 consist of discrete Cu6(μ3-I)6 hexagon prisms, which are coordinated with bridging 1,4-bis(phenythio)butyne ligands via Cu–S bonds. Contrary to the other rare literature-known examples of metallopolymers incorporating Cu6X6 SBUs as connecting nodes, the Cu⋯Cu interactions of 2 [2.8484(6) Å] are markedly shorter, being close to the sum of the Van der Waals radii of two Cu atoms (∼2.8 Å). The photophysics of these compounds, which exhibit reversible luminescence thermochromism, has been investigated in detail. The solid-state luminescence spectra of 1 and 2 feature at room temperature intense emissions around 560 and 555 nm, respectively. The luminescence properties of the unusual Cu6(μ3-I)6 hexagon prism motif are rationalized by means of DFT and TDDFT computations.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH2SPh
CCH2SPh ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH2SPh}3]n (2). The SBUs of luminescent 2 consist of discrete Cu6(μ3-I)6 hexagon prisms, which are coordinated with bridging 1,4-bis(phenythio)butyne
CCH2SPh}3]n (2). The SBUs of luminescent 2 consist of discrete Cu6(μ3-I)6 hexagon prisms, which are coordinated with bridging 1,4-bis(phenythio)butyne 

 Please wait while we load your content...
Please wait while we load your content...