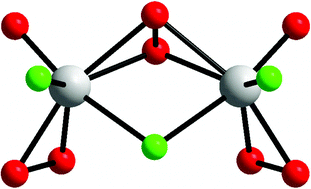
Dinuclear fluoro-peroxovanadium(v) complexes with symmetric and asymmetric peroxo bridges: syntheses, structures and DFT studies†
Abstract
Two new dinuclear fluoro peroxovanadium(V) complexes, Cs3[V2O2(O2)4F]·H2O (1) and Cs3[V2O2(O2)3F3]·2HF·H2O (2), were prepared and characterized by

 Please wait while we load your content...
Please wait while we load your content...