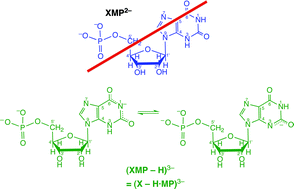
The four acidity constants of threefold protonated xanthosine 5′-monophosphate, H3(XMP)+, reveal that in the physiological pH range around 7.5 (X − H·MP)3− strongly dominates and not XMP2− as commonly given in textbooks and often applied in research papers. Therefore, this nucleotide, which participates in many metabolic processes, should be addressed as xanthosinate 5′-monophosphate as is stated in this critical review. Micro acidity constant schemes allow quantification of intrinsic site basicities. In 9-methylxanthine nucleobase deprotonation occurs to more than 99% at (N3)H, whereas for xanthosine it is estimated that about 30% are (N1)H deprotonated and for (X − H·MP)3− it is suggested that (N1)H deprotonation is further favored, especially in macrochelates where the phosphate-coordinated M2+ interacts with N7. The formation degree of these macrochelates in the (X − H·MP·M)− species of Co2+, Ni2+, Cu2+, Zn2+ or Cd2+ amounts to 90% or more. In the monoprotonated (M·X − H·MP·H)± complexes, M2+ is located at the N7/[(C6)O] unit as the primary binding site and it forms macrochelates with the P(O)2(OH)− group to about 65% for nearly all metal ions considered (i.e., including Ba2+, Sr2+, Ca2+, Mg2+); this indicates outer-sphere binding to P(O)2(OH)−. Finally, a new method quantifying the chelate effect is applied to the M(X − H·MP)− species, stabilities and structures of mixed-ligand complexes are considered, and the stability constants for several M(X − H·DP)2− and M(X − H·TP)3− complexes are estimated (112 references).

 Please wait while we load your content...
Please wait while we load your content...