The OH + D2→ HOD + D angle–velocity distribution: quasi-classical trajectory calculations on the YZCL2 and WSLFH potential energy surfaces and comparison with experiments at ET = 0.28 eV
Abstract
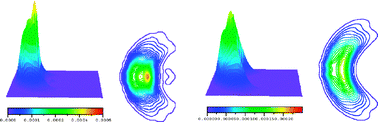
The angle–velocity distribution (HOD) of the OH + D2 reaction at a relative translational energy of 0.28 eV has been calculated using the quasi-classical trajectory (QCT) method on the two most recent potential energy surfaces available (YZCL2 and WSLFH PESs), widely extending a previous investigation of our group. Comparison with the high resolution experiments of Davis and co-workers (Science, 2000, 290, 958) shows that the structures (peaks) found in the relative translational energy distributions of products could not be satisfactorily reproduced in the calculations, probably due to the classical nature of the QCT method and the importance of quantum effects. The calculations, however, worked quite well for other properties. Overall, both surfaces led to similar results, although the YZCL2 surface is more accurate to describe the H3O PES, as derived from comparison with high level ab initio results. The differences observed in the QCT calculations were interpreted considering the somewhat larger anisotropy of the YZCL2 PES when compared with the WSLFH PES.

 Please wait while we load your content...
Please wait while we load your content...