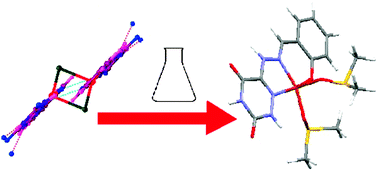
A lot of effort has been put into the synthesis of copper complexes with superoxide-dismutase (SOD) activity because of their potential pharmaceutical applications. In this work, we study a model for these so-called SOD mimics (SODm), namely a copper complex of 6-(2-hydroxy-benzaldehyde) hydrazono-as-triazine-3,5-dione, which shows an extremely high SOD-like activity in solution. X-Ray diffraction reveals that the complex adopts a di-copper structure in the solid state. However, in solution, the chloride bridges are broken, forming a mono-copper center as follows from UV/Vis absorption and electron paramagnetic resonance (EPR) experiments. Using pulsed EPR techniques in combination with DFT (density functional theory) computations, the electronic structure of the complex in solution is analyzed in detail and related to its high SOD activity. The structure–activity analysis serves to orient further synthetic efforts to obtain the optimum geometry around the metal essential for SOD-like activity.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...