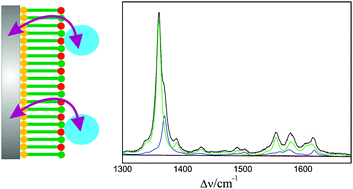
The anionic soluble heme protein cytochrome b562 was electrostatically immobilised on Ag electrodes coated with positively charged self-assembled monolayers of amino-terminated alkanethiols. The structure of the heme pocket, the redox equilibria, and the electron transfer dynamics were studied by stationary and time-resolved surface enhanced resonance Raman spectroscopy, complemented by cyclic voltammetry measurements of the interfacial redox process. The conformational and redox equilibria of the immobilised protein are compared to those of the cationic heme protein cytochrome c immobilised on negatively charged electrode coatings. Similarities and differences can be rationalised in terms of the respective electric fields at the interfaces of amino- and carboxyl-terminated electrode coatings. The heterogeneous electron transfer rate of cytochrome b562 only slightly increases with decreasing thickness from ca. 20 to 11 Å, implying that the electron tunneling is not the rate-limiting step. In contrast to cytochrome c on carboxyl-terminated monolayers, this behaviour cannot be attributed to protein re-orientation gating the heterogeneous electron transfer. Instead, it may reflect the interplay between interprotein electron transfer and heterogeneous electron transferviaprotein orientations exhibiting particularly high tunneling probabilities for the electron exchange with the electrode.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...