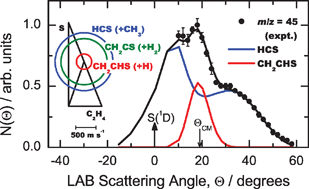
The reaction between excited sulfur atoms, S(1D), and the simplest alkene molecule, ethene, has been investigated in a complementary fashion in (a) crossed-beam dynamic experiments with mass spectrometric detection and time-of-flight (TOF) analysis at a collision energy of 37.0 kJ mol−1, (b) low temperature kinetic experiments ranging from room temperature down to 23 K, and (c) electronic structure calculations of stationary points and product energetics on the C2H4S singlet potential energy surface. The rate coefficients for total loss of S(1D) are found to be very large (ca. 4 × 10−10 cm3 molecule−1 s−1) down to very low temperature indicating that the overall reaction is barrier-less. From laboratory angular and TOF distributions at different product masses, three competing reaction channels leading to H + CH2CHS (thiovinoxy), H2 + CH2CS (thioketene), and CH3 + HCS (thioformyl) have been unambiguously identified and their dynamics characterized. Branching ratios have also been estimated. These studies, which exploit the capability of producing intense supersonic beams of sulfur S(3P,1D) atoms and measuring rate coefficients down to very low temperature, offer considerable promise for further dynamical investigations of other sulfur atom reactions of particular relevance to combustion and atmospheric chemistry.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...