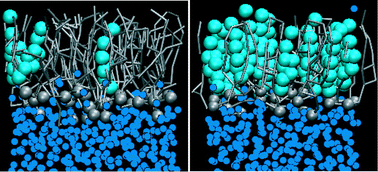
We have investigated the effect of cholesterol concentration on the properties of lipid monolayers at air/water interfaces at low surface tensions. This is of interest for understanding the properties and function of lung surfactant monolayers. Lung surfactant lines the gas exchange interface in the lungs and dramatically reduces the surface tension, thereby preventing lung collapse and decreasing the work associated with breathing. Changes in the lipid composition of lung surfactant, particularly an increase in cholesterol concentration, can result in inhibition of its function, as in the case of acute respiratory distress syndrome. We have used molecular dynamics simulations with both atomistic and coarse-grained force fields to study lipid monolayers containing DPPC, POPG and cholesterol in molecular ratios of 8 : 2 : 1 and 4 : 1 : 4 at surface tensions of 40, 20 and 0 mN m−1 at 310 K. These mixtures model the lipid component of lung surfactant at normal (∼9%) and elevated (∼44%) cholesterol concentration. We have characterised the structural and dynamic properties of these monolayers and calculated the free energy for transfer of each lipid from its equilibrium position in the monolayer into water and into air (vacuum). The results show that at low surface tensions an increase in cholesterol concentrations leads to formation of a liquid-condensed phase with low area compressibility, which is in agreement with experimental findings.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...