Self-association-free dimeric cinchona alkaloid organocatalysts: unprecedented catalytic activity, enantioselectivity and catalyst recyclability in dynamic kinetic resolution of racemic azlactones†
Abstract
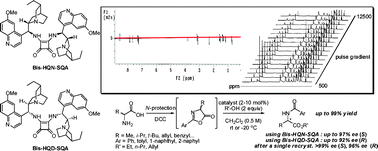
Self-association-free, bifunctional, squaramide-based dimeric

 Please wait while we load your content...
Please wait while we load your content...