Electrocyclization of cis-dienals in organic synthesis: a new and versatile synthetic method for the preparation of aryl- and heteroaryl-fused coumarins†
Abstract
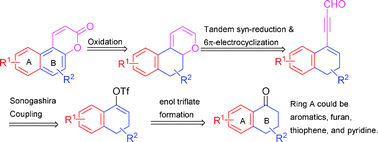
Benzo-, furo-, thieno-, and pyrido[f]coumarins were prepared by generating the 2H-pyran-2-one moieties from the oxidation of the corresponding 2H-pyran rings, which were formed in situ from the electrocyclization of cis-dienals.

 Please wait while we load your content...
Please wait while we load your content...