Chiral Pd aqua complex-catalyzed asymmetric C–C bond-forming reactions: a Brønsted acid–base cooperative system†
Abstract
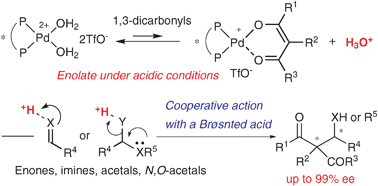
Chiral cationic Pd aqua complexes can function as acid–base
- This article is part of the themed collection: Selective Catalysis for Organic Synthesis

 Please wait while we load your content...
Please wait while we load your content...