The synthesis of novel core-substituted naphthalene diimides via Suzuki cross-coupling and their properties†
Abstract
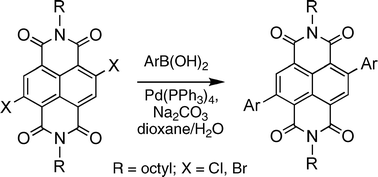
This paper reports the efficient core-substitution of halogenated naphthalene diimides utilizing the Suzuki cross-coupling reaction to install various

 Please wait while we load your content...
Please wait while we load your content...