A new USP Class VI-compliant substrate for manufacturing disposable microfluidic devices
Abstract
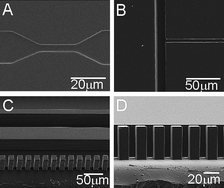
As microfluidic systems transition from research tools to disposable clinical-diagnostic devices, new substrate materials are needed to meet both the regulatory requirement as well as the economics of disposable devices. This paper introduces a UV-curable polyurethane-

 Please wait while we load your content...
Please wait while we load your content...