Structure of fluoride-containing bioactive glasses
Abstract
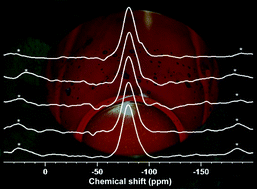
Fluoride prevents dental cavities, stimulates bone mineralisation and decreases the melting temperature of glasses and is therefore an interesting component of bioactive glasses for use as dental or orthopaedic biomaterials. However, when designing new glass compositions, the structural role of fluoride in the glass needs to be better understood. We have characterised a glass series in the system SiO2–P2O5–CaO–Na2O with increasing concentrations of CaF2. Network connectivity was fixed at 2.13 by adding CaF2 while the ratio of all other components was kept constant. 19F and

 Please wait while we load your content...
Please wait while we load your content...