Chemo-enzymatic synthesis of N-alkyloxaziridines mediated by lipases and urea-hydrogen peroxide†
Abstract
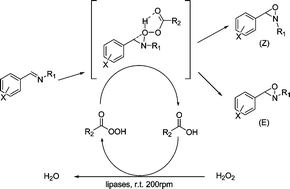
Chemo-enzymatic oxidation of various N-alkylimines mediated by lipases produced the corresponding E- or a mixture of E- and Z-N-oxaziridines with moderate to very high yields (>99%) and good to excellent selectivities (70–100%) within 30 min to 6 h in various organic

 Please wait while we load your content...
Please wait while we load your content...