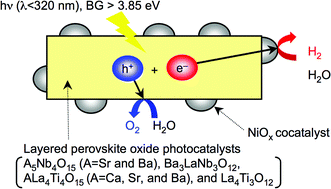
Photophysical and photocatalytic properties of A5Nb4O15 (A = Sr and Ba), Ba3LaNb3O12, ALa4Ti4O15 (A = Ca, Sr, and Ba), and La4Ti3O12 with layered perovskite structures, in which a plane in parallel with (111) of a simple perovskite structure was exposed at interlayer, were investigated. These oxides were obtained by a polymerizable complex method at 973–1473 K though only A5Nb4O15 (A = Sr and Ba) were prepared by a solid state reaction even at 1673 K. The shapes of these complex metal oxides were plate-like derived from the perovskite layered structure. These band gaps were estimated to be 3.7–4.1 eV from the onsets of diffuse reflection spectra. These oxides showed photoluminescence at 77 K. These oxides loaded with NiO cocatalysts showed activities for water splitting under UV irradiation. NiOx/BaLa4Ti4O15 and NiOx/Ba5Nb4O15 showed the highest activities among the titanates and niobates tested in the present study. NiOx/BaLa4Ti4O15 and NiOx/Ba5Nb4O15 gave 15% and 17% of quantum yields at 270 nm, respectively. Photocatalytic activities of ALa4Ti4O15 (A = Ca, Sr, and Ba) strongly depended on the alkaline earth metal ion. Pt, Au, Ni, and PbO2 were selectively photodeposited on basal or edge plane of the BaLa4Ti4O15 plate-like powder while these were randomly loaded on CaLa4Ti4O15. It was suggested that this difference in the surface property was the one of the important factors affecting photocatalytic ability for ALa4Ti4O15 (A = Ca, Sr, and Ba).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...