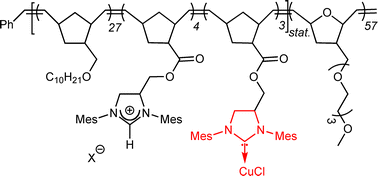
Ring-opening metathesis polymerization has been used for the synthesis of the amphiphilic block-copolymer poly(M1-co-M3)-b-poly(M2) from the hydrophilic monomer 5-{2-[2-(2-methoxy-ethoxy)-ethoxy]-ethoxymethyl}-7-oxabicyclo[2.2.1]hept-2-ene (M2), and the hydrophobic monomers endo,exo-5-decyloxymethyl-bicyclo[2.2.1]hept-2-ene (M1) and 1,3-di(1-mesityl)-4-{[(bicyclo[2.2.1]hept-5-en-2-ylcarbonyl)oxy]methyl}-4,5-dihydro-1H-imidazol-3-ium carboxylate (M3). Poly(M1-co-M3)-b-poly(M2) was loaded with Cu and the resulting CuI-loaded polymer poly(M1-co-M3)-b-poly(M2)–Cu was used for a series of Cu-catalyzed reactions under micellar conditions, i.e. for the [3 + 2] cycloaddition of azides to alkynes and for carbonyl hydrosilylation reactions. Under such micellar conditions, the polymer-bound Cu-catalyst was found to be an efficient catalyst for all reactions investigated. Turn-over numbers (TONs) in cycloaddition reactions were in the range of 200–375, those in hydrosilylation reactions ∼2000 allowing for Cu-loadings of 0.05 mol% with respect to substrate.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...