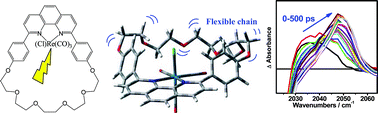
Two sets of supramolecular rhenium carbonyl-phenanthroline complexes were prepared: fac-[Re(Cl)(CO)3(N,N)] and fac-[Re(Etpy)(CO)3(N,N)]+, where N,N is 2,9-di-anisyl-1,10-phenanthroline (dap) or two related macrocyclic ligands, where the two anisyl groups are connected by a polyether chain of different length and rigidity (m27, m30), which wraps around and above the equatorial Re(CO)2 group. The excited-state character and relaxation dynamics of these complexes were investigated by picosecond time-resolved IR spectroscopy in the spectral region of C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O stretching vibrations, ν(CO). The results were interpreted with the aid of DFT and TD-DFT calculations of the molecular structures and electron-density redistribution upon excitation. [Re(Cl)(CO)3(phen-macrocycle)] in CH2Cl2 have the same type of lowest excited state as analogous acyclic phen or bpy complexes, that is a mixed Re(CO)3→phen and Cl→phen MLCT/XLCT, together with some ππ*(phen) IL character. Its relaxation dynamics are qualitatively similar to those of phen or bpy complexes. However, relaxation of [Re(Cl)(CO)3(m30)] shows a slow kinetics component (∼22 ps) which arises from confined local solvent molecules and/or from conformational movements of the flexible m30 polyether ring. In contrast, attaching anisyl groups at the 2 and 9 phen positions in [Re(Etpy)(CO)3(phen-macrocycle)]+ effectively “freezes” excited-state relaxation in MeCN, regardless of the presence of the macrocyclic ring. The lowest excited triplet state has a mixed MLCT/IL character. Restricting the solvent access to the excited chromophore clearly affects both the character and dynamics of the lowest excited state.
O stretching vibrations, ν(CO). The results were interpreted with the aid of DFT and TD-DFT calculations of the molecular structures and electron-density redistribution upon excitation. [Re(Cl)(CO)3(phen-macrocycle)] in CH2Cl2 have the same type of lowest excited state as analogous acyclic phen or bpy complexes, that is a mixed Re(CO)3→phen and Cl→phen MLCT/XLCT, together with some ππ*(phen) IL character. Its relaxation dynamics are qualitatively similar to those of phen or bpy complexes. However, relaxation of [Re(Cl)(CO)3(m30)] shows a slow kinetics component (∼22 ps) which arises from confined local solvent molecules and/or from conformational movements of the flexible m30 polyether ring. In contrast, attaching anisyl groups at the 2 and 9 phen positions in [Re(Etpy)(CO)3(phen-macrocycle)]+ effectively “freezes” excited-state relaxation in MeCN, regardless of the presence of the macrocyclic ring. The lowest excited triplet state has a mixed MLCT/IL character. Restricting the solvent access to the excited chromophore clearly affects both the character and dynamics of the lowest excited state.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O stretching vibrations, ν(CO). The results were interpreted with the aid of DFT and TD-DFT calculations of the molecular structures and electron-density redistribution upon excitation. [Re(Cl)(CO)3(phen-macrocycle)] in CH2Cl2 have the same type of lowest excited state as analogous acyclic phen or
O stretching vibrations, ν(CO). The results were interpreted with the aid of DFT and TD-DFT calculations of the molecular structures and electron-density redistribution upon excitation. [Re(Cl)(CO)3(phen-macrocycle)] in CH2Cl2 have the same type of lowest excited state as analogous acyclic phen or 

 Please wait while we load your content...
Please wait while we load your content...