Ruthenium based catalysts for olefinhydrosilylation: dichloro(p-cymene)ruthenium and related complexes†
Abstract
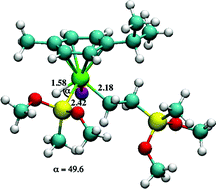
We report our third and final investigation into the use of ruthenium based compounds for catalyzing the hydrosilylation of
- This article is part of the themed collection: The synergy between theory and experiment

 Please wait while we load your content...
Please wait while we load your content...