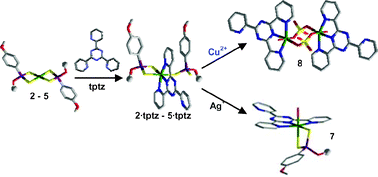
The reactions between tptz and differently substituted dithiophosphonato [Ni(ROpdt)2] [ROpdt = (RO)(4-MeOC6H4)PS2−; R = Et (2); Pr (3); i-Pr (4); Bu (5)] and dithiophosphato [Ni((EtO)2PS2)2] (6) NiII complexes have been investigated, and the characterisation of the resulting neutral mixed complexes (2·tptz)-(6·tptz) is reported. In all these complexes, tptz forces one of the two dithiophosphonato/dithiophosphato ligands to behave as a monodentate ligand, a coordination mode rarely found in analogous NiII phosphorodithioato complexes. A comparison has been performed between the Ni–S bond distances of the new complexes and those of isologous dithiophosphonato, dithiophosphato and dithiophosphito NiII square-planar complexes, and of their penta- and hexa-coordinated adducts. The results, also supported by DFT calculations, are discussed and explained in terms of the structural trans-effect (STE). The reactivity of 2·tptz towards AgNO3 and CuSO4 to yield the complex [Ni(EtOpdt)(tptz)(H2O)]NO3 (7), and the dimer [(Ni(tptz)(μ-SO4)(H2O)]2·6H2O (8), respectively, is consistent with the proposed bonding models.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...