Stereoelectronic effects in a homologous series of bidentate cyclic phosphines. A clear correlation of hydroformylation catalyst activity with ring size†
Abstract
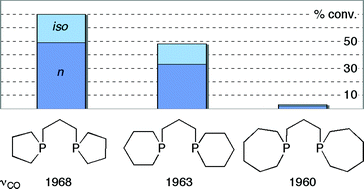
The homologous series of diphosphines (CH2)n−1P(CH2)3P(CH2)n−1 where n = 5 (L5), 6 (L6), or 7 (L7) have been synthesized from the corresponding PhP(CH2)n−1. Treatment of [PtCl2(cod)] with L5–7 gave the 6-membered chelates cis-[PtCl2(L5–7)], the crystal structures for which reveal that L5–7 have very similar steric bulk and bite angles. Treatment of [Rh2Cl2(CO)4] with L5–7 gave the binuclear trans-[Rh2Cl2(CO)2(μ-L5–7)2] with syn and anti orientations of the CO and Cl

 Please wait while we load your content...
Please wait while we load your content...